| I. Acoustic Surveys |
When deploying ultrasonic detectors to survey bats, workshop participants agreed that there are some fundamental details which must be included when reporting results:
1. EQUIPMENT:
2. AMBIENT CONDITIONS:
3. ANALYSIS:
| II. Describing Habitat "Batscapes" |
When habitats are described in terms of their plant communities and by their structure in both the horizontal and vertical planes, it becomes possible to predict which bat species will be able to utilize the habitat. This is grounded in the fact that different habitats not only provide variable food resources, but they also influence how a bat travels through the landscape: a bat's echolocating abilities are intrinsically linked to the ecological conditions and each species has a tolerance limit to function in: (a) highly cluttered space (e.g, dense forest); (b) background-cluttered space (e.g., edge habitat); or (c) uncluttered space (open airspace).
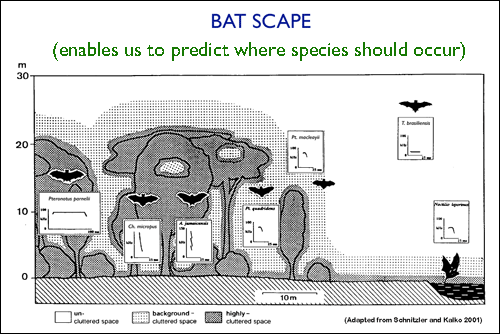 |
In a hypothetical vegetation plot, quantifiable characteristics of a "Batscape" include:
| Fleming, Geiselman, and Kress' (2009) Appendix 2 New World bat-pollinated species listed by plant family is an invaluable starting-point for predicting which plant species will be pollinated by bats in Jamaica. |
Given that bats can easily commute > 10 km from a roosting cave to terrestrial feeding areas (and recognizing that the intervening corridor must meet their acoustic travel requirements), all landuses under Forestry Department's "Forest" Classification (e.g., Closed Broadleaf, Short Open Dry, Mangrove etc., but with the exception of Caribbean Pine and Eucalyptus plantations) should be presumed to be good "Batscapes" for forest-dependent species.
The default for native forest habitats should be: assume pollinating-, seed-dispersing, and insect-eating forest-dependent bats are present unless an exhaustive survey shows otherwise.
| III. Survey Guidelines |
Identical to bird and other faunal surveys where the objectives of the survey define what will be appropriate survey methodologies and sampling protocols, bat surveys have to be designed with the recognition that there is no single technique that can detect all species of bats equally. Their numerous morphological, physiological, and behavioural adaptations of sensory and motor systems permit bats to have access to a wide range of habitats and resources at night. These adaptations completely influence how we can detect bats and how they can detect us and our equipment.
 CONCLUSIONS & RECOMMEDATIONS from the field workshops and seminar
CONCLUSIONS & RECOMMEDATIONS from the field workshops and seminar
(with supplement from peer-reviewed literature):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) Ultrasonic bat detectors are most effective for detecting bats which use lower frequency and narrow bandwidth calls (e.g., aerial insectivores, such as the family Molossidae) and
bats that emit Constant Frequency (e.g., Pteronotus parnellii) or Quasi-Constant Frequency/Frequency Modulated calls with moderate bandwiths (e.g., the family Mormoopidae).
|
|
(b) Ultrasonic bat detectors are poor at detecting species which emit very high frequencies or Frequency Modulated calls with wide bandwidths. This includes gleaning insectivores and nectarivores in
the family Phyllostomidae ("whispering" bats) and insectivores in the family Natalidae.
|
|
(c) Exceptions to the "whispering" Phyllostomidae rule are the Jamaican Fruit Bat (Artibeus jamaicensis) and the endemic, tree-roosting Jamaican Fig-eating Bat
(Ariteus flavescens ). Artibeus jamaicensis can emit an intense call of ~ 100 decibels (measured 10 cm from its mouth) and
radio-transmittered Ariteus flavescens could be detected acoustically
to about 3-5 meters. Coupled with Mormoopidae, establishing baseline Nightly Activity Indices and monitoring for changes in activity patterns is feasible and practical with
acoustic surveys for these species in forest and forest-edge habitats.
|
|
(d) Distance-detection range with bat detectors will always be limited by the acoustic properties of the calls emitted by the bats: if a proposed development structure is going to occupy
the airspace 50 m above the ground, then the bat detector must be elevated to ensure that airspace is correctly evaluated.
|
|
(e) Capture of Bats: While detection of species presumed to be rare or uncommon may be increased by employing multiple methods, such as ground-based and canopy-based mist nets and harp traps (Pio et al. 2011
Meyer et al. 2011), these techniques also have biases. For example, they are limited to surveying only their area-of-interception and mist nets fluttering in even a slight breeze
(Beaufort #2; blowing < 5 km / hr) may be detected and avoided, esp. by insectivorous bats.
|
| ALERT: Mist nets can (will) be LETHAL to wildlife if they are deployed by untrained persons. Because diurnal birds as well as bats can be captured at dusk and dawn and nocturnally-active wetland birds (e.g., shorebirds, night-herons, etc.), owls, nightjars, and potoos may be captured throughout the night, seminar participants who have been trained to handle both birds and bats advise that only those persons who have been permitted by international banding / ringing schemes should be allowed to deploy mist nets unsupervised in Jamaica until national skills-certification is established. This is both for the safety of the wildlife as well as for the safety of the person handling the animals. There are many opportunities for professional training at numerous bird banding stations in North America and Europe. The North American Banding Council's Instructor's Guide has detailed descriptions of the minimum levels to which someone must be trained to safely use mist nets. Persons who have been trained to handle birds but not bats should start their training by referring to the Bat Workers' Manual, 3rd ed. published by Joint Nature Conservation Committee (UK). Although general descriptions are given for the safe deployment and extraction of bats from mist nets, bird banders need to be trained so they do not break the bat's forearm while working the net free from the elbow and carpal joints. Of additional usefulness, the manual describes the licensing system for those needing to work with bats in the UK. For example, a Class Licence Level 3 is required to deploy mist nets and acoustic lures while a Class Licence Level 4 is required to deploy harp traps. Persons also should be aware that all birds in Jamaica are protected unless they appear on the Second Schedule of the Wild Life Protection Act. In the absence of a permit issued by NEPA, their capture in mist nets, even if incidental ("by-catch") is an offense against the Act and can result in a fine of up-to JD 100,000 or one year in jail.
|
|
|
|
|
| Windsor Research Centre would like to extend a special thank you to all field workshop and seminar participants for their contributions to what we hope will lead to improved conservation of
bats, the ecosystem services they provide, and their terrestrial and subterranean habitats.
Please if you have any questions or additional comments. |
Useful References
- - 2000. Biophysical Inventory Manual. Trees for Tomorrow Project, Phase II. Joint project between Forestry Department (Ministry of Agriculture, GoJ) and Tecsult International (Canada).
Clare, E.L., H.R. Goerlitz, V.A. Drapeau, M.W., M.W. Holderied, A.M. Adams, J. Nagel, E.R. Dumont, P.D.N Herbert, and M.B. Fenton. 2014. Trophic niche flexibility in Glossophaga soricina: how a nectar seeker sneaks an insect snack. Functional Ecology v28. http://dx.doi.org/10.1111/1365-2435.12192.
Emrich, M.A., E.L. Clare, W.O.C. Symondson, S.E. Koenig, and M.B. Fenton. 2013. Resource partitioning by insectivorous bats in Jamaica. Molecular Ecology 23: 3648-3656.
Fleming, T.H., C. Geiselman, and W.J. Kress. 2009. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104: 1017-1043.
Hayward, C.E. 2013. DNA barcoding expands dietary identification and reveals dietary similarity in Jamaican frugivorous bats. Electronic Thesis and Dissertation Repository. Paper 1697. http://ir.lib.uwo.ca/etd/1697
Kay, E. 2001. Observations on the pollination of Passiflora penduliflora. Biotropica 33:709-713.
Lobova, T.A., C.K. Geiselman, and S.A. Mori. 2009. Seed dispersal by bats in the Neotropics. The New York Botanical Garden, NY, USA
MacSwiney, M.C., F.M. Clarke, and P.A. Racey. 2008. What you see is not what you get: the role of ultrasonic detectors in increasing inventory completeness in Neotropical bat assemblages. Journal of Applied Ecology 45: 1364–1371.
Meyer, C.F.J., L.M.S. Aguiar, L.F. Aguirre, J. Baumgarten, F.M. Clarke, et al. 2011. Accounting for detectability improves estimates of species richness in tropical bat surveys. Journal of Applied Ecology 48: 777-787.
Meyer, C.F.J., L.M.S. Aguiar, L.F. Aguirre, J. Baumgarten, F.M. Clarke, et al. 2015. Species undersampling in tropical bat surveys: effects on emerging biodiversity patterns. Journal of Animal Ecology, 24: 113-123.
Petrov B. 2008. Bats – methodology for environmental impact assessment and appropriate assessment. A manual for developers, environmental experts and planning authorities. National Museum of Natural History-BAS, 88 p.
Pio, D.V., F.M. Clarke, I. Mackie, and P.A. Racey. 2010. Echolocation calls of the bats of Trinidad, West Indies: is guild membership reflected in echolocation signal design? Acta Chiropterologica 12: 217-229.
Rodrigues, L., L. Bach, M.-J. Dubourg-Savage, J. Goodwin & C. Harbusch. 2008. Guidelines for consideration of bats in wind farm projects. EUROBATS Publication Series No. 3 (English version). UNEP/EUROBATS Secretariat, Bonn, Germany, 51 pp.
Schnitzler, H.-U. and E.K.V. Kalko. 2001. Echolocation by insect-eating bats. BioScience 51: 557-569.
Click here to return to the Main Page of the Seminar.
Acknowledgements:
Funding for the seminar was provided by:
 |

