| Fact Sheet posted 28th October 2014 Last Updated: 10th November 2014 |
Disclaimer: This Fact Sheet contains the professional opinion of Windsor Research Centre (WRC) and is based on the references listed at the bottom of the page. This document is not intended to be an authoritative statement or to be an authority about the origin and transmission of Ebola. The author (Dr. Susan Koenig - Wildlife Ecologist) and WRC are not liable for any errors or omissions in this document.
WRC encourages readers to consult medical authorities, such as Médecins Sans Frontières (MSF) and World Health Organization (WHO) for advice on preventing Human-to-Human transmission of Ebola.
We take seriously all alien pathogens and parasites -- not only those which cause diseases in their native hosts, but also those which appear benign. Island populations are often "immunologically naïve": oceans are good barriers to the movement of terrestrial wildlife and the micro-organisms which travel with them, so when alien pathogens (such as viruses or bacteria) are introduced, (either intentionally or accidentally), islander immune systems can be overwhelmed until antibodies develop to fight the pathogen...or an individual dies. The arrival of Chikungunya virus (Chik-V) to Jamaica in 2014 highlights how susceptible island populations are when a new virus arrives.
One article which helped enormously in preparing this Fact Sheet was:
Olival, K.J. and D.T.S. Hayman. 2014. Filoviruses in Bats: Current Knowledge and
Future Directions. Viruses. Apr 2014; 6(4): 1759–1788.
Published online Apr 17, 2014. doi: http://dx.doi.org/10.3390/v6041759
We also recommend Sonia Shah’s excellent article “The Spread of New Diseases: The Climate Connection”, which explains how microbial pathogens can jump ("spillover") from wildlife to people, particularly when humans invade a forest. The transmission of disease-causing organisms from wildlife to humans is called "zoonosis".
For a discussion about Ebola and dogs, visit the website of the Jamaica Veterinary Medical Association (JVMA).
| FACT SHEET: Ebolavirus & Wildlife | ||
Llovius virus, detected in 2002 in the Iberrian Peninsula (France, Spain, and Portugal), is provisionally classified as a distinct genus, "Cuevavirus", and species "Lloviu cuevavirus". |
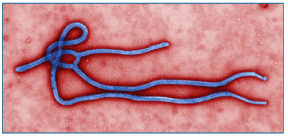 | |
The fifth species of Ebolavirus (Reston ebolavirus) occurs in Asia: it does not cause disease in humans but it causes disease in domesticated pigs and can kill non-human primates (notably, macaques). It remains uncertain whether Zaire ebolavirus also occurs in Asia or whether there exists an intermediate form between Zaire and Reston ebolavirus. | ||
The 2007 Ebola outbreak in Democratic Republic of Congo corresponded to increased bat activity associated with bat migration and hunting -- large-scale slaughter -- of fruit bats for bushmeat prior to the outbreak. The suspected index case for the current outbreak of Zaire ebolavirus in West Africa is a 2-year old child, who died in Meliandou, Guéckédou prefecture, southeastern Guinea, in December 2013. This location is more than 2,000 km away from all previous Ebola outbreaks. The 2014 Ebola outbreak in Democratic Republic of Congo (DRC), which peaked in August, began on 26th July when a woman fell ill a few days after butchering a primate of an unknown arboreal species. The primate had been found dead by her husband. The variant strain of Zaire eboluvirus matches to previous Ebola outbreaks in DRC (notably the 1995 Kikwit outbreak) and is genetically distinct from the Zaire ebolavirus variant circulating in West Africa since December 2013 / early 2014: the two outbreaks are not epidemiologically connected. | ||
Species of Rousette fruit bats (Roursettus spp.) in Bangladesh and China have been found to be seropositive (i.e., they had developed antibodies) for both Reston and Zaire ebolavirus, raising concern that there is perhaps a genetic intermediate between these two viral species or that Zaire ebolavirus is present not only in Africa but also in Asia. This second concern -- Zaire ebolavirus present in Asia -- is worrying because Old World fruit bats also are eaten in Asia. |
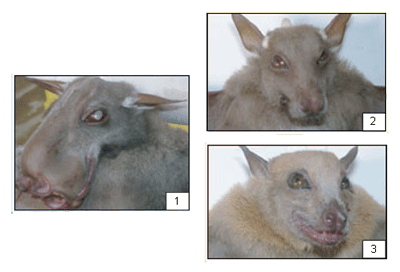 African (Old World) Fruit Bats Order: Chiroptera Family: Pteropodidae 1. Hypsignathus monstrosus 2. Epomops franqueti 3. Myonycteris torquata |
|
Artibeus jamaicensis ranges from the Lower Florida Keys, through the Greater and Lesser Antilles to Grenada (but not in Trinidad and Tobago). It also occurs from central Mexico to the northwest of South America, west of the Andes Mountains. Six subspecies are recognized. Although taxonomists are wrangling over the assignment of species and subspecies designations for the mainland populations, it is recognized that the subspecies Artibeus jamaicensis jamaicensis is restricted to the Lesser and Greater Antilles, except for Cuba and the Bahamas which have a different subspecies Artibeus jamaicensis parvipes. Molecular analyses show that there isn't regular movement of individuals between the mainland and the islands. In Jamaica, this species normally roosts in caves, but it also will roost in trees and occassionally in buildings. Within caves, a single male will have a harem of a few females. During the night, it may rest temporarily in buildings, particularly when it is near a fruit tree: this behaviour can lead to potential conflicts with humans. Ariteus flavescens is endemic to Jamaica: it occurs nowhere else in the world. It also is the only species in the genus Ariteus - it is our most genetically isolated and unique bat species. The species typically roosts in trees, including coconut trees in areas of agro-forestry; it will roost in caves opportunistically when ripe fruit is nearby. We've seen bats roosting individually and in small groups of about 10 individuals: it's not what one would describe as a highly social and gregarious species (i.e., not good if you're a virus needing high densities of a host, to ensure good rates-of-contact for transmission). |
  Neotropical Fruit Bats Order: Chiroptera Family: Phyllostomidae A. Artibeus jamaicensis B. Ariteus flavescens |
|
What is the potential risk that Jamaican bats harbour Ebolavirus?
There has been no testing for Filoviruses (including the genus Ebolavirus) in Jamaican bats, so we must assess the risk based on
information from: (a) other surveillance programmes; (b) the phylogeny (i.e. genetic relationships) and biogeography (i.e., distributions) of Filoviruses and of Old World & New World bats; and
(c) the current understanding of Ebolavirus transmission routes -- both wildlife-to-wildlife and wildlife-to-human.
| Based on the currently-available information about the distribution of the virus Family Filoviridae and of the five virus species in the genus Ebolavirus -- notably that they (or antibodies to them) have been detected in free-ranging wildlife ONLY in the Old World and no surveillance programmes have reported Filoviridae in any wildlife (including bats) in the New World -- |
| The probability that Jamaican bats harbour Ebolavirus is VERY LOW. |
| But given the fatal seriousness of Ebola, follow what is always good advice: |
| Avoid direct handling of wildlife (including blood, tissue products, and other body fluids).
Don't touch or eat any animal carcass you find. Don't eat bats (Order Chiroptera) or primates (Order Primates). |
The very low risk that Jamaican bats carry an Ebolavirus does not mean that they couldn't harbour other types of blood-borne pathogens and parasites found in New World bats -- without a surveillance programme, we can't say.
The one disease of which everyone should be aware is Histoplasmosis, a respiratory disease
caused by the fungus Histoplasma capsulatum. It grows in soils enriched by animals droppings, including those from bats and birds (e.g., poultry farms).
The U.S. Centers for Disease Control and Prevention (CDC) provides information about
Histoplasmosis prevention and treatment .
How can we protect Jamaican bats (and ourselves) from alien Ebolaviruses?
The natural geographical isolation of Jamaica, surrounded by the Caribbean Sea, leads researchers to predict that our island wildlife has fewer pathogens and parasites compared
to mainland ecosystems, leaving them immunologically-naïve to micro-organisms commonly found in mainland wildlife. To protect our native and endemic wildlife:
|
|
|
|
|
|
|
|
|
|
References Used to Prepare This Webpage:
Animals (Diseases and Importation) Act, 1948. (Amended 1969). Government of Jamaica. Available electronically through Ministry of Justice, GoJ:
http://www.moj.gov.jm/laws/animals-diseases-and-importation-act
Animals Diseases (Importation) Control Regulations, 1948. (Amended 1989). Government of Jamaica.
Barrette, R.W., S.A., Metwally, J.M. Rowland, L. Xu, S.R. Zaki, S.T. Nichol, P.E. rollin, J.S. Towner, W.J. Shieh, B. Batten, et al. 2009. Discovery of swine as a host for the Reston ebolavirus. Science: 325(5937): 204-206. doi: 10.1126/science.1172705.
Carroll S.A., J.S. Towner, T.K. Sealy, L.K. McMullan, M.L. Khristova, F.J. Burt, R. Swanepoel, P.E. Rollin, and S.T. Nichol. 2013. Molecular evolution of viruses of the family Filoviridae based on 97 whole-genome sequences. Journal of Virology. 87:2608–2616. doi: 10.1128/JVI.03118-12.
Larsen, P.A., S.R. Hoofer, M.C. Bozeman, S.C. Pederson, and H.H. Genoways. 2007. Phylogenetics and phylogeography of the Artibeus jamaicensis complex based on Cytochrome-b DNA sequences. Mammology Papers: University of Nebraska State Museum. Paper 53. http://digitalcommons.unl.edu/museummammalogy/53
Leroy, E.M., P. Rouquet, P. Formenty, S. Souquiere, A. Kilbourne, J-M. Froment et al. 2004. Multiple Ebola virus transmission events and rapid decline of Central African wildlife. Science 303: 387-390.
Leroy, E.M., B. Kumulungui, X. Pourrut, P. Rouquet, A. Hassanin, P. Yaba, A. Delicat, J.T. Paweska, J-P. Gonzalez. and R. Swanepoel. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438:575-576. doi:10.1038/438575a
Maganga, G.D., J. Kapetshi, N. Berthet, B.K. Ilunga, F. Kabange, P. M. Kingebeni, V. Mondonge, et al. 2014. Ebola virus disease in the Democratic Republic of Congo. New England Journal of Medicine 2014; 141021130018004 doi: 10.1056/NEJMoa1411099.
Negredo A, G. Palacios G, S. VázquezMorón, F. González F, H. Dopazo, et al. 2011.
Discovery of an EbolavirusLike Filovirus in Europe. PLoS Pathogens 7(10): e1002304.
Published online Oct 20, 2011. doi:10.1371/journal.ppat.1002304
Olival, K.J. and D.T.S. Hayman. 2014. Filoviruses in Bats: Current Knowledge and
Future Directions. Viruses. Apr 2014; 6(4): 1759–1788.
Published online Apr 17, 2014. doi: http://dx.doi.org/10.3390/v6041759
Ortega, J. and I. Castro-Arellano. 2001. Artibeus jamaicensis. Mammal Species No. 662. Published by American Society of Mammalogists.
Pigott, D.M., N. Golding, A. Mylne, Z. Huang, A.J. Henry, D.J. Weiss, O.J. Brady, et al. 2014. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife 2014;3:e04395. doi: http://dx.doi.org/10.7554/eLife.04395
Pourrut, X., A. Delicat, P.E. Rollin, T.G. Ksiazek, J-P. Gonzalez, and E.M. Leroy. 2007. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species.. Journal of Infectious Diseases 196 (Supplement 2): S176-S183. doi: 10.1086/520541.
Pourrut X., M. Souris, J.S. Towner, P.E. Rollin, S.T. Nichol, J.P. Gonzalez, and E.M. Leroy. 2009. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infectious Diseases. 2009:9 doi: 10.1186/1471-2334-9-159.
Shah, S. 2009. The spread of new diseases: the climate connection. On-line article, Yale University.
Simmons, N. B. 2005. Order Chiroptera. In: Mammal species of the World: a taxonomic and geographic reference, Third Edition (D. E. Wilson and D. M Reeder, eds.). Smithsonian Institution Press. Online database: www.vertebrates.si.edu/msw/mswcfapp/msw/index.cfm
Taylor, D.J., K. Dittmar, M.J. Ballinger, and J.A. Bruenn. 2011. Evolutionary maintenance of filovirus-like
genes in bat genomes. BMC Evolutionary Biology 11: 336
Published online Nov 17, 2011. doi:10.1186/1471214811336
Taniguchi, S., S. Watanabe, J.S. Masangkay, T. Omatsu, T. Ikegami. P. Alviola, N. Ueda, K. Iha, H. Fujii et al. 2011. Reston ebolavirus antibodies in bats, the Philippines. Emerging Infectious Diseases 17: 1559-1560. doi:10.3201/eid1708.101693.
Walsh, P.D., T. Breuer, C. Sanz, D. Morgan, and D. Doran-Sheehy. 2007. Potential for Ebola transmission between gorilla and chimpanzee social groups. American Naturalist 169: 684-689.
Weingartl, H.M., C. Embury-Hyatt, C. Nfon, A. Leung, G. Smith, and G. Kobinger. 2012. Transmission of Ebola virus from pigs to non-human primates. Scientific Reports 2: Article number: 811. doi:10.1038/srep00811
Whitfield, J. 2003. Ape populations decimated by hunting and Ebola virus. Nature 422: 551 | doi:10.1038/422551a
Virus Pathogen Database and Analysis Resource (ViPR) online database: Filoviridae [http://viprbrc.org/brc/home/home.spg?decorator=filo]
Yuan, J., Y. Zhang, J. Li, Y. Zhang, L-F. Wang, and Z. Shi. 2012. Serological evidence of ebolavirus infections in bats, China.
Virology Journal 9: 236.
Published online Oct 13, 2012. doi:10.1186/1743422X9236

