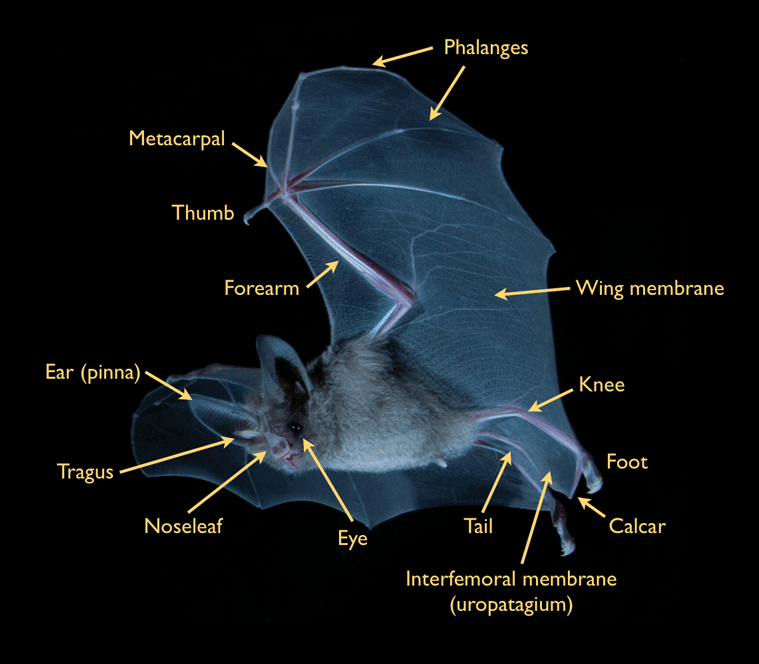
Bats dominate the native mammalian diversity on Jamaica. Although known locally as 'Ratbats,' bats actually are more closely related to primates than they are to rodents. Their scientific classification, Chiroptera, comes from the Greek roots cheir (hand) and pteron (wing). As the name implies, the wing of a bat is a highly modified hand, with elongated fingers and forearm over which a thin, elastic skin is stretched. This design enables bats to travel by powered flight - the characteristic which distinguishes bats from all other mammals.
Bats have been evolving for about 52 million years into what today are more than 1300 unique species. On Jamaica, there are 21 species representing 6 families (Genoways et al. 2005). Three additional species are known from the fossil record (Tonatia bidens, Brachyphylla nana, and Mormoops megalophylla). At least four of our extant species are endemic to Jamaica and a further six have their ranges restricted entirely to the insular Caribbean.
Ten of Jamaica's 21 bat species are obligate cave dwellers, including three of the four endemics. Bat colonies occupy less than 17% of caves documented island-wide and nearly 1/3 of these bat caves are recorded in the environs of Cockpit Country.
Species richness in the majority of documented caves is low, with nearly 60% of bat caves occupied by fewer than four species. Fewer than 10 caves account for all the known colonies of some of the rarest species, such as Phyllonycteris aphylla. Several caves in Cockpit Country (e.g., Windsor Great Cave [for which WRC is the Agent of the owner, WWF-UK], Marta Tick Cave) are notable for their high diversity, which includes representation of insectivores, frugivores, and specialized nectivores. Others, such as Wallingford Sink and Bristol Cave, are noted for the collapse of their bat faunas over the past 70 years, quite likely due to excessive disturbance associated with guano harvesting and tourism (McFarlane 1986) -see threats to caves.
Of species that are not obligate cave-dwellers (i.e., roost in tree hollows or man-made structures), the endemic Jamaican Red Bat (Lasiurus degelidus) is of particular scientific and ecological interest as this is the only genus of bat in which there are commonly more than two young per birth (Nowak 1994). The majority of bats produce only a single pup per year. This low annual reproductive performance leaves bat populations vulnerable if non-volant pups die when nursery caves are disturbed.
The largest bat colonies are dominated by the mustached bats of the genus Pteronotus, of which three species occur in Jamaica. The largest species, Parnell's Mustached Bat (Pteronotus parnellii), is the only bat in the New World to use a Constant-Frequency (as opposed to Frequency-Modulated), echolocation signal. In the past it was collected extensively for bioacoustic laboratory research (e.g., Kobler et al. 1985, Henson and Henson 1991, Kanwal et al. 1994). Ecological information for wild populations is rather limited (see Goodwin 1970, Genoways et al. 2005, and Emrich et al. 2013) and we have no demographic data (annual reproduction and survival rates; longevity) for this species.
Indeed, there are few published studies on the ecology and habitat requirements of any of Jamaica's bats. Most published accounts have been based on very limited collecting, often strictly for taxonomic or biogeographic research (e.g. Dávalos and Eriksson 2003), for studying acoustic calls under controlled circumstances (e.g. Kṏssl et al. 1999), or for determining the species composition of bat-occupied caves (NEPA 2011). We often refer to ecological studies conducted in Cuba (e.g., Silva Taboada 1979; Macina et al. 2013) and Puerto Rico (Gannon et al. 2005; Rolfe 2011) or the mainland (Handley et al.1991) to make predictions for Jamaican bats.
Diet
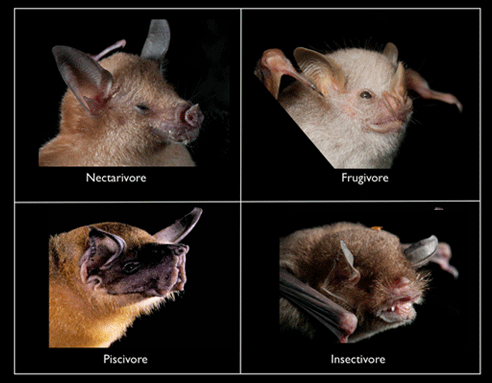 There are four major feeding guilds on Jamaica:
There are four major feeding guilds on Jamaica:
- Nectarivores (4 species): feed on nectar and pollen (but they can also be good at eating small fruits and sneaking insect snacks [Clare et al. 2013.)
- Frugivores (2 species): feed on fruit and pollen (with incidental insects, which bore inside the fruit)
- Piscivores (1 species): fish (but also aquatic crustaceans and winged insects)
- Insectivores (14 species): flying and crawling insects and other arthropods such as spiders
- Aerial-hawkers: when insects are flying around, the bat has to track moving prey and must be able to constantly update information about the insect's position as it (the insect) undertakes evasive maneuvers
- Gleaners: when prey is stationary, the bat typically detects prey-generated signals
Sound-scape
When bats are foraging, the amount of "clutter" in the environment is one of the most important factors which constrains them. "Clutter" can
be described by the proximity of the desired prey item to the clutter of vegetation or the ground. Such clutter represents perceptual and mechanical problems.
Perceptually, bats are constrained by their sensory capabilities (e.g., echolocation, passive listening, vision, and olfaction) to detect, classify, and locate
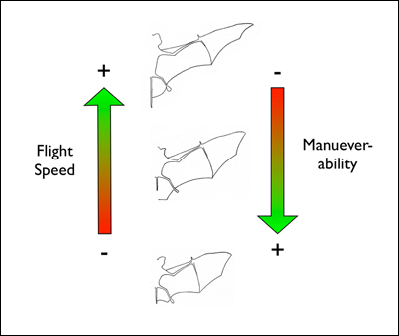
food which is located within a cluttered environment. Mechanically, bats are constrained by their motor capabilities, such as flight speed and maneuverability.
So, for example, bats which hunt in a the understory of a closed-canopy forest need to be highly maneuverable to avoid colliding with vegetation while trying to
intercept flying insects.
Schnitzler and Kalko (1998) describe acoustic habitats and foraging modes as:
- Uncluttered space - aerial hunter: bats which feed on flying insects in open spaces, high above the ground, and far from vegetation; the only collision risk is with another bat (or a larger avian predator!)
- Background-clutted space: bats which forage along edges of vegetation, near the ground, or near a water surface have to recognize an insect echo from the echoes of background vegetation (i.e. distinguish food from non-food) and then be able to avoid collision with all non-food targets (e.g. leaves, stems, rocks). Within this background-cluttered space, each species will be further adapted to hunt for aerial insects or glean for stationary prey.
- Highly-cluttered space: bats which forage in dense forest or very close to leaves or the ground not only have to be highly maneuverable to avoid collision with all the abiotic clutter, but they face a big challenge when echoes from the clutter actually mask the echoes from a prey item. Just as in "background-cluttered space" habitat, "highly-cluttered space" insectivores are specialized to either hunt for aerial insects or glean for stationary prey. Nectarivores and frugivores have to avoid colliding with the stems and leaves of the plants they are visiting
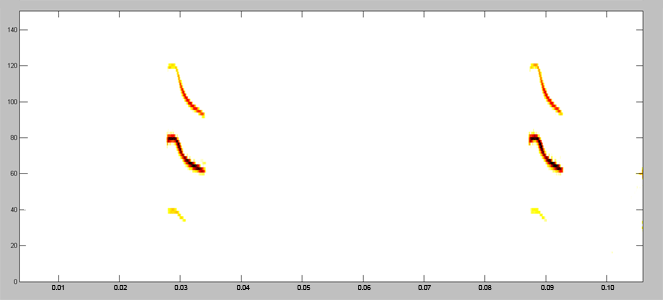
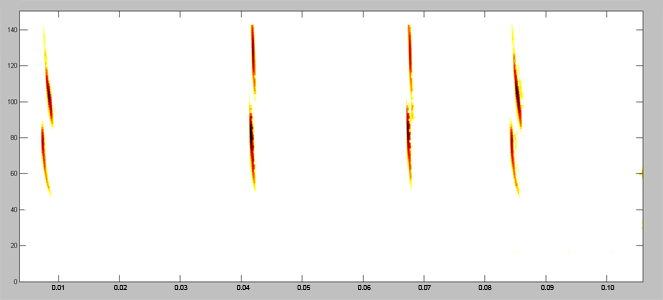
Acoustically, aerial hawkers tend to produce long, low frequency calls, which carry far through open space. Gleaners tend to produce higher frequency calls, typically very short, steep Frequency Modulated (FM) calls. Higher frequencies attenuate (lose energy) more quickly but they provide better resolution of environmental clutter.
By examing wing morphology, call characteristics, and facial features, we can predict the diet and in which habitats one would expect to find each of Jamaica's 21 bat
species hunting and feeding. For example:
| (A) Open-space, aerial-hawking insectivore - Tadarida brasiliensis |
| (B) Background-cluttered space, aerial-hawking insectivore - Pteronotus quadridens |
| (C) Background & highly-cluttered space, nectarivore - Glossophaga soricina |
 |
 |
 |
 |
 |
|
 |
 |
The economic importance of bats
Insectivorous bats consume enormous quantities of nocturnal insects, including many that are harmful to crops and pests to humans. For example, during the non-breeding season, an insectivorous bat will consume at least 50% of its body mass each night in insects. During the breeding season, lactating females can consume up-to 120% of their body mass nightly. So, for example, if a cave roost contains 100,000 individuals of Parnell's Mustached Bats (average weight = 14 grams), they would consume nearly 285 tonnes of insects each year. The overwhelming majority of this species' diet is composed of moths (Lepidoptera; Emrich et al. 2013), so by consuming so many adult moths, the bat is controlling the leaf-damaging caterpillar stage of these insects.
Fruit-eating bats are the most important seed-dispersing mammals in the tropics. Because they generally defecate in flight, seeds are dispersed away from the mother tree, thus increasing a seedling's chances of survival. In forest regeneration studies in Africa, Mexico, and Asia, bats were responsible for dispersing 75-95% of all seeds, with birds, primates, and other animals accounting for the balance (Medellín and Gaona 1999 and references therein, Vermeulen and Whitten 1999).
Nectar-feeding bats, along with some fruit bats that visit flowers, pollinate thousands of bat-dependent tropical trees and shrubs. Among commercially-valuable plants, bats are responsible for pollinating mango, guava, avocado, fig, and cashew.
Guano is rich in phosphate and used as fertilizer and organic mulch. Commercial harvest must be assessed VERY carefully to ensure disturbance does not cause the bats to abandon the cave, with the recognition that other suitable cave environs may not be available in the area (i.e. the bats will die). As the Agent of WWF-UK, Windsor Research Centre does not allow guano harvesting from Windsor Great Cave.
Scientific, aesthetic and cultural value of bats
Echolocation and bioacoustic studies dominate the scientific research of Jamaica's bats. Pteronotus parnellii are particularly used because they emit at a constant frequency of about 60KHz and use the doppler effect (the frequency shift of echoes) to "see" their prey. Their ear passage has thus evolved with a particularly long section which is tuned to the 60KHz region, making the researchers' task easier.
The evening emergence of large bat colonies enables visitors to observe bats without disturbing roosting and nursery colonies within the caves.
Unfortunately, Jamaicans generally don't think very highly of "ratbats", particularly when a colony of Pallas' Mastiff Bats (Molossus molossus) gets established in a ceiling or when a Jamaican Fruit Bat (Artibeus jamaicensis) carries some fruit inside a house, to feed in a nice, safe corner of a room. Fortunately, these nuisance issues can be handled safely and efficiently without causing any direct harm to the bats. Please see our Frequently-Asked-Questions page for a few simple solutions.
| HEALTH CAUTION FOR CAVE EXPLORATION IN JAMAICA (Fincham 1997) |
|
|
Literature Reviewed
Dávalos, L.M. and R. Eriksson. 2003. New and noteworthy records from ten Jamaican bat caves. Caribbean Journal of Science 39: 140-144.
Emrich, M.A., E.L. Clare, W.O.C. Symondson, S.E. Koenig, and M.B. Fenton. 2013. Resource partitioning by insectivorous bats in Jamaica. Molecular Ecology 23: 3648-3656.
Fenton, M.B. and N.B Simmons. 2014. Bats: a world of science and mystery. University of Chicago Press, Illinois, USA. pp. 305.
Fincham, A. 1997. Jamaica Underground. Unversity of the West Indies Press, Mona, Jamaica. pp. 447.
Gannon, M.R., A. Kurta, A. Rodriguez-Duran, and M.R. Willig. 2005. Bats of Puerto Rico. University of the West Indies Press, Mona, Jamaica. pp. 239.
Genoways, H.H., R.J. Baker, J.W. Bickham, C. J. Phillips. 2005. Bats of Jamaica. Museum Texas Tech Univ. Texas, USA. pp 155.
Goodwin, R.E. 1970. The ecology of Jamaican bats. J. Mammology 51: 571-579.
Handley, C.O., Jr, D.E. Wilson, and A.L Garnder (eds.). 1991. Demography and natural history of the Common Fruit Bat Artibeus jamaicensis on Barro Colorado Island, Panama. Smithsonian Institution Press, Washington, D.C.
Henson, N.M. and O.W. Henson, Jr. (1991). Specializations for sharp tuning in the mustached bat: The tectorial membrane and spiral limbus. Hearing Research 56: 122-132.
Kanwal, J.S., S. Matsumura, K. Ohlemiller, and N. Suga. 1994. Analysis of acoustic elements and syntax in communcation sounds emitted by mustached bats. Journal of the Acoustical Society of America 96:1229-1254.
Kobler, J.B., B.S. Wilson, O.W. Henson, Jr., and A.L. Bishop (1985). Echo intensity compensation by echolocating bats. Hearing Research 20: 99-108.
Kṏssl, M., F Mayer, G. Frank, M. Faulstich, and I.J. Russell. 1999. Evoluationary adaptations of coclear function in Jamaican mormoopid bats. Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology 185:217-228.
McFarlane, D.A. 1986. Cave bats in Jamaica. Oryx 20: 27-30.
Mancina, C.A., L. Garcia-Rivera, and B.W. Miller. 2012. Wing morphology, echolocation, and resource partitioning in syntopic Cuban mormoopid bats. Journal of Mammology 95: 1308-1317.
Medellín, R.A. and O. Gaona. 1999. Seed dispersal by bats and birds in forest and distrubed habitats in Chiapas, Mexico. Biotropica 31: 478-485.
National Environment and Planning Agency. 2011. Bat Management Plan for Jamaica 2012 – 2017. Ecosystems Management Branch, NEPA, Government of Jamaica.
Nowak, R.M. 1994. Walker's Bats of the World. The Johns Hopkins University Press, Baltimore, Maryland.
Pregill, G.K., R.I. Crombie, D.W. Steadman, L.K. Gordon, F.W. Davis, and W.B. Hilgartner. 1991. Living and Late Holocene fossil vertebrates, and the vegetation of the Cockpit Country, Jamaica. Atoll Research Bulletin No. 353. National Museum of Natural History, Smithsonian Institution, Washington, D.C.
Rolfe, A. K. 2011. Diet of Three Mormoopid Bats (Mormoops blainvillei, Pteronotus quadridens, and Pteronotus portoricensis ) on Puerto Rico. Master's Theses and Doctoral Dissertations. Paper 349.
Schnitzler, H.-U. and E.K.V. Kalko. 1998. How echolocating bats search and find food. Pp. 183-196. In (eds. T.H. Kunz and P.A. Racey) Bat Conservation and Biology. Smithsonian Institution Press, Washington, D.C.
Silva-Taboada, G. 1979. Los murciélogos de Cuba. Habana. Editorial de la Academia de Ciencias de Cuba. pp. 425.
Vermeulen, J. and T. Whitten. 1999. Biodiversity and cultural property in the management of limestone resources. The World Bank, Washington, D.C.

